Kate Giffin, Claire Shudde, and Nick Jänne
Dear Readers,
In our fourth edition of EquilibriUM, we asked our contributors to explore their everyday wonders – commonplace experiences in life that, despite being so regular, provoke curiosity, examination, and awe.
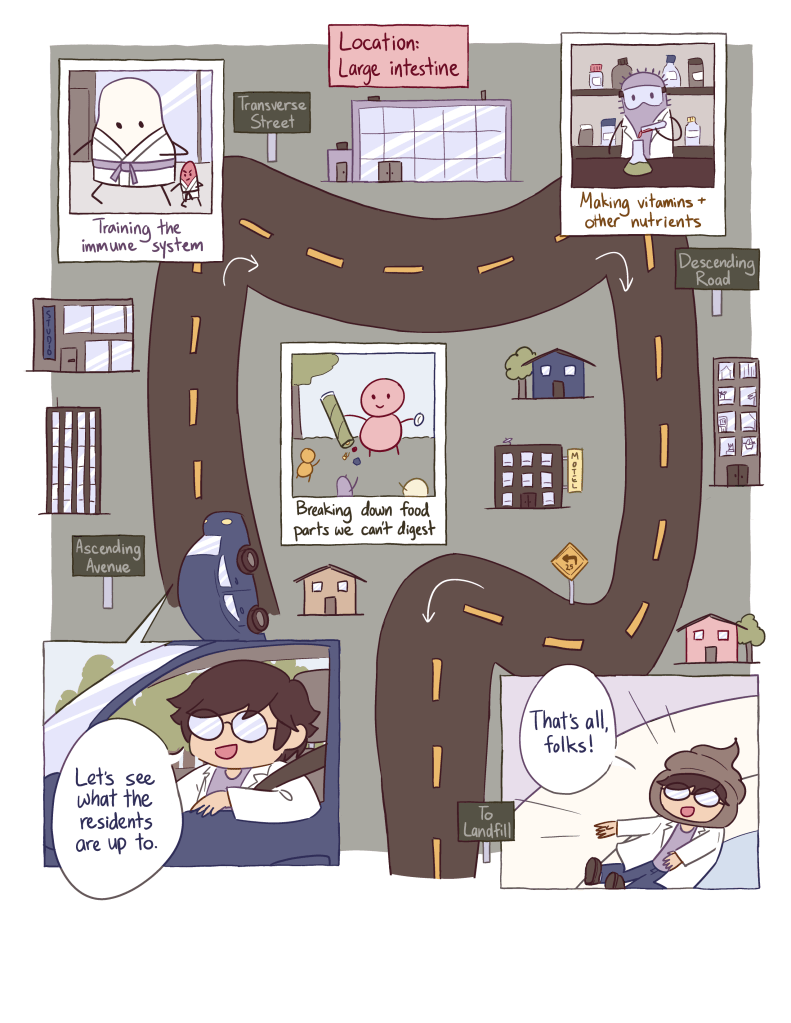
The beauty of an everyday wonder extends far beyond itself. It is an opportunity to learn something new, to share a story, or to create with what has already been created. Most surprising in this edition of our magazine is the degree to which the everyday wonders presented are both wholly disconnected from one another in subject and form, but unified in turning a small experience into something beautifully enormous. The creators found wonders in the most everyday experiences, from the sidewalks we walk upon (Sidewalk Cracks) to our perception of colors (Invisible Rainbow) and the glass that splits the rainbow (Invisible Cradle), then tied it all together with both science and art (Natural Lines of Fracture). They explored our food, from the battle to keep crops healthy (Fighting in the Fields) to cultural and sensory neuroscience (Science of Spice) to the journey of food through the body (The Marvelous Gastrointestinal Tract). They dived deep into protecting our bodies, both our internal defenses (Life on the Edge) and cutting-edge biomedical advances (Unlikely Allies). To add to this year’s magazine, each contributor had to do three distinct things: wonder, create, and share. We would like to take this opportunity to expand on the process of all three.
Wondering is a human impulse, something that is fundamentally designed for and performed by everybody – of all ages and walks of life. Some may wonder more than others, some may have internalized that they don’t wonder at all, or that wondering isn’t “for them,” but we all experienced the immersive joy of being curious.
Many wonders are left unanswered, but many too, sprout a drive for artistic, academic, or whimsical creation. You will find a wide variety of mediums and formats in this magazine that highlight our authors and illustrators’ unique methods of creation – paintings, comics, short stories, explainers, and more. To create is to turn an idea into your idea. But know this: the ownership and pride that comes with creation is in the choice of getting started, not the medium you use.
Some people choose to share their creations with the world, and others don’t. Either way is fine by us, but we all three can attest to the delight of enjoying the works of others. It is precisely why we are so committed to this magazine as members of the editorial staff. Sharing your work with the world is akin to the gentle touch of “I like this, and I thought you might, too.” It is an invitation to see the wonders of the world through another person’s eyes. And what a beautiful thing that is if we can hold onto it.
Wonder, create, and share.These three behaviors are not special to us because they fit the requirements of our magazine this year, rather, they underpin a vast majority of what connects us to one another. Importantly, they also stand as the reasons why many pursue scientific research – at least before the year 2025. More and more, we are watching the foundation of science in the United States be shattered by a sledgehammer in the name of efficiency.
We cannot have Nobel laureates and Fields medalists without little astronauts and paleontologists searching for extraterrestrial or prehistoric life in the backyard with the dog. We cannot have life-saving medications, world-saving climate solutions, or peaceful communities if science is denied and scientists are silenced. Research funding, immigrants, and support for scientists from all backgrounds enables the scientific advancements that come from wondering, creating, and sharing. We cannot stand on the world stage as curious, impactful researchers devoted to the common good once “curious,” “common,” and “good” have been stripped from the script. These words are being pried loose by billionaires and lawmakers still struggling to muster the wonder required to understand the very science they threaten. Safety, including physical, mental, and financial security, is required to be able to wonder and explore science to the fullest. While this edition is overwhelmingly joyful, we want to take a moment to emphasize the existential threats facing our work as scientists and community members. There is much work to be done to defend these wonders.
We hope that by reading our contributors’ work, you exercise the courage to participate. We believe that wondering, creating, and sharing, is and should be for everybody, as it always has been. If you find an idea in our magazine, on your trip home from work or school tomorrow, or anywhere else that you may be, ask yourself the following: where did this come from? What could I do with it? Who could I tell this to? Results to all three will be surprising. We also hope that as you encounter science in the news, you think back to us and how the bright minds of tomorrow are reliant on the policy of today.
Thank you for reading and supporting our magazine. As long as we are able to, we will continue to pour fuel on the beautiful curiosities of others in the pursuit of wondering, creating, and sharing.
Among other things, Kate Giffin (center) is a PhD candidate in neuroscience. In the lab, she studies how severe infections can lead to long-term brain issues like dementia. She is
passionate about telling scientific stories through unexpected genres, particularly poetry, to expand the way people think about science and the world. When Kate is not marveling
at the everyday wonder of the brain, she is probably outside marveling at some strange plant.
Claire Shudde (right) is a Ph.D. candidate in pharmacology studying the everyday wonder of the immune system and how it can fight cancer and autoimmune disease. Outside of
the lab, she enjoys dancing, reading, and editing a friend’s novel. She hopes people leave this magazine with more awe for the world around them.
Nick Jänne (left) is a PhD student in Robotics, researching how robots can improve their scope of capabilities in the real world by learning from humans. He also hopes to one day
build human habitats on the Moon and Mars using a team of robots and humans. Nick received his Bachelors of Computer Engineering degree from the University of Michigan in
2023, and has a passion for reading and writing on the next generation of artificial intelligence.
Photography by Paola Medina-Cabrera