Live Blogger: Christian Greenhill
Editor: Emily Eberhardt
This piece was written live during the 6th annual RNA Symposium: Towards our Future of RNA Therapeutics, hosted by the University of Michigan’s Center for RNA Biomedicine. Follow MiSciWriter’s coverage of this event on Twitter with the hashtag #umichrna.
A tiny “genetic patch” can be used to cure common diseases that affect millions of people. At the 6th Annual RNA Symposium, Dr. Michelle Hastings gives us a taste of what goes on in her lab in the Windy City at the Chicago Medical School. The @HastingsLab focuses on designing tiny “genetic patches,” or oligonucleotides, to repair genetic processes that lead to severe neurodegenerative diseases, such as Usher syndrome, Batten’s disease, and cystic fibrosis. Over the last decade, Dr. Hastings’ work has led to numerous patents and FDA-approved therapies to improve symptoms associated with these diseases. Her work demonstrates that RNA is a powerful platform and target for future therapeutics.
At the RNA Symposium, Dr. Hasting brings us into the conversation by describing how genes in our DNA are expressed through an intricate messaging system. Genes determine our traits, such as our hair color, skin color, and height. Genes are composed of an alphabet of four letters called nucleotides: A, T, C, and G. This messaging system provides instructions to keep our body healthy. Health problems can occur if there is a spelling error or a ‘mutation’ in the gene.
Making Sense of Antisense
Dr. Hastings’ title is “Splice-Modulating Antisense Oligonucleotides for the Treatment of Disease”. This sounds complicated, so let’s break it down. Some ‘mutations’ can cause a problem with the splicing of a gene. When there is a splicing problem, the messenger RNA cannot give the correct instructions for proper production of hormones, proteins, and regulatory cellular processes. Dr. Hastings’ point is that a splicing problem can be fixed with a tiny synthetic molecule, called an antisense oligonucleotide, or ASO. Antisense means the tiny molecule is ‘complementary’ to the messenger RNA strand, and can therefore bind to it. Oligo means ‘small’, because ASOs are made up of relatively few (usually fewer than 25) nucleotides. An ASO is a small string of DNA or RNA letters that can stick to the messenger RNA.
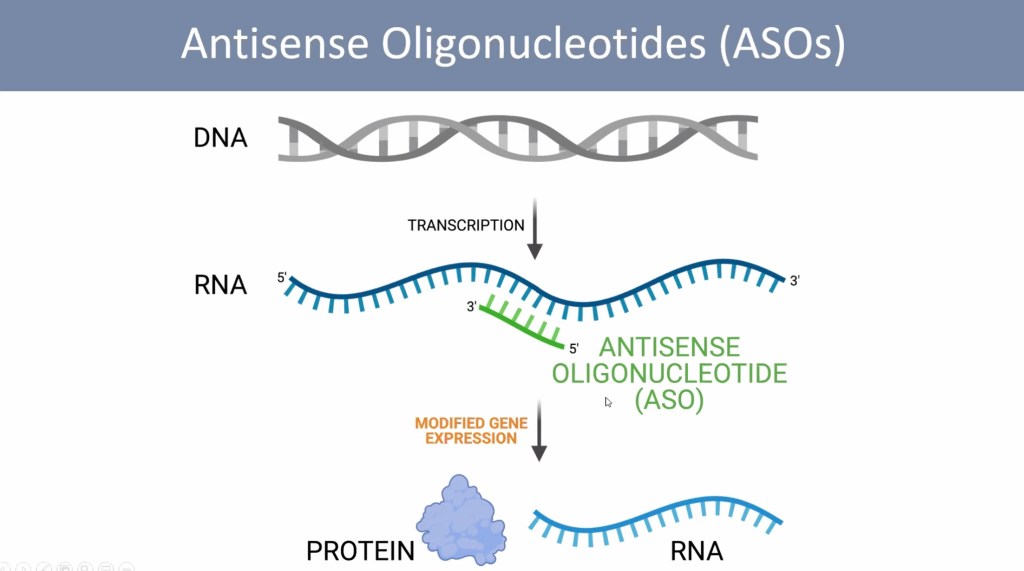
Curing Blindness and Deafness with ASOs
Dr. Hastings draws our attention to Usher syndrome (US), a condition that leads to vision and hearing loss, caused by a mutation in the USH1C gene. The mutated gene is recessive, meaning that it is expressed in people whose parents are both carriers of the mutation.
Dr. Hastings presents a striking video to the audience at the RNA Symposium. The video shows the behavior of two mice: one mouse has normal USH1C genes and the other has mutated USH1C genes. In the 10-15 second video clip, the normal mouse walks in straight paths, while the mutant mouse runs in circles constantly, due to a loss of awareness because it cannot see or hear. Dr. Hastings hypothesizes that if a particular splicing site on the USH1C gene could be “patched up” or “blocked” by a tiny ASO, the resulting protein can form correctly and the severity of the condition could improve. What the audience then saw in the video was remarkable. The response of the mutant mouse improved as soon as 6 weeks after the ASO was administered, showing that the mutant mouse had gained a sense of awareness. The video was a very compelling demonstration for ASOs potential as a therapeutic!
ASO Switching – The First Drug for Muscular Atrophy!
Dr. Hastings discusses the same concepts in the context of Batten’s Disease, cystic fibrosis, and muscular atrophy, leaving us with a positive outlook towards the future!
An audience member asks “In relation to industry and pharma companies… how can academic labs make the greatest impact in this field?” Dr. Hasting responds with: “There are thousands of genes in our DNA. And within these thousands of genes, there are so many possible mutations. Industry and pharmaceutical companies cannot cover it all. Therefore, there is a lot of potential. Bridging the gap between academics and industry is an important path forward for development and progress in RNA therapeutics”.

A Champion for Students and Female Scientists
Michelle Hastings, PhD, is a Professor and the Director of the Center for Genetic Diseases at the Chicago Medical School at Rosalind Franklin University of Medicine and Science. Her work clearly demonstrates that antisense technology can modulate gene expression pathways associated with many diseases. On her website, Dr. Hastings illuminates the work of women and pioneers. Such ideas challenge systems that may be viewed as fundamentally biased against the advancement of women and minorities and call for mentors to be held accountable for successes of their graduate students and postdocs. Dr. Hastings was recognized by the Illinois Science and Technology Consortium as a 2019 Researcher to Know.
