By Jimmy Brancho
The fuel source of the future might be a lot more familiar than you think.
Plenty of people are excited about solar energy’s replacing fossil fuels. Harvesting, processing, and burning fossil fuels is a major contributor to environmental pollution and political conflict. Could we reduce those problems by using solar energy instead? Industry seems to think so; the most recent National Renewable Energy Laboratory Data Book statistics show that electricity output from solar installations has grown continually throughout the last decade – nearly 75% from 2011 to 2012 alone.
But what happens when the sun goes down? Are you just supposed to not binge Netflix at midnight?
One potential answer to that question is called solar water splitting, which harvests hydrogen gas from liquid water for use as a fuel.
Solar, like all intermittent power sources, is “use it or lose it” – your calculator works when the light shines on it, but when it’s in the dark, you’re back to mental math. Solar energy storage refers to any process by which that temporary sunlight is converted to another energy source which can be used on-demand. Using a solar cell to charge a battery is an example of solar energy storage.
Another good example is to use sunlight to power a chemical reaction and generate a “solar fuel.” If that sounds familiar, that’s because plants have been doing it for billions of years with photosynthesis. Artificial photosynthesis – that is, storing solar energy in a chemical fuel outside of a leaf – is a general term that could refer to a number of different reactions.
As an example of artificial photosynthesis, let’s take a closer look at water splitting. Water is non-toxic, environmentally safe, cheap, and abundant. Could it be a practical fuel source?
At first glance, water splitting looks fairly easy. Water has two hydrogen atoms and an oxygen atom. During water splitting, two molecules of water get decomposed into their elements – two molecules of hydrogen gas (H2) and one molecule of oxygen gas (O2). Importantly, water splitting is a reduction/oxidation (“redox”) reaction, relying on the transfer of electrons between molecules. As water is split, the formation of O2 excludes some electrons. Those electrons are stored in hydrogen and become a source of energy. Burning hydrogen is the reverse of water splitting and is also a redox reaction. Because redox reactions involve electron transfer, they can be used for storing electrical energy and releasing it on-demand.
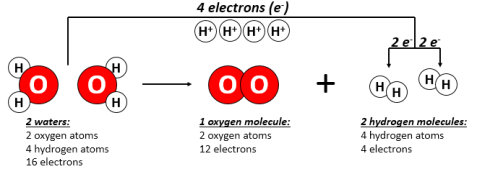
However, water splitting isn’t as easy as it appears. Water is a very stable molecule, which has turned out to be a good thing for living organisms. Splitting it takes a lot of energy input. Getting all the hydrogen out of just a shot glass full of water would kill your iPhone 5 battery more than 30 times – a month’s worth of battery use. But you don’t get anywhere if you split water with electricity alone. Splitting water by using energy input from fossil fuels – electricity, for example – just moves the energy from fossil fuels into hydrogen. That doesn’t solve our reliance on fossil fuels. But what if it could be done using solar energy?
How exactly can water splitting store solar energy? In solar water splitting, there are two common methods. In one scheme, a photovoltaic solar cell just like the one on your old calculator is hooked up to an electrolyzer, an electrical device that uses the voltage from the solar cell to split water. In the second, photochemical water splitting, the sun shines on a catalyst material and excites it, giving it enough energy to generate hydrogen and oxygen. These two methods can also be combined in a method known as photoelectrochemical water splitting, such that photochemical water splitting is helped along by a little extra electrical input.

In all three of these configurations, solar energy helps a reaction that requires lots of energy input. When we collect the molecular hydrogen product from this reaction, we also collect the solar energy stored in its chemical bonds. We can then package the hydrogen up, process it, and use it as a fuel or as an ingredient for other chemical reactions. When needed, the hydrogen is burned, the stored energy gets released, and water is regenerated. Voila! Solar energy on-demand!
None of the three is necessarily better than the other. One approach to solar energy storage might be more applicable to powering your car, and another might be better for powering a huge facility that keeps the lights on in your entire town. For example, a challenge for cars running on hydrogen is that compressed hydrogen gas doesn’t store a lot of energy for the amount of space it takes up. Using solar energy to charge a compact, rechargeable battery is probably better. However, for a stationary power plant, space is less important, so using water splitting to generate hydrogen could work more cost-effectively than a battery.
Solar water splitting is an exciting area of science, not only because it could provide conveniently available energy without greenhouse gas emissions, but because it sits at the intersection of chemistry, physics, and materials science – which makes for fun and challenging research. Some huge strides have already been made, but we are a long way from seeing hydrogen-powered devices become mainstream. As with any industry, cost-efficiency is the bottom line. Cheap water splitting devices that operate for a long time are still elusive, but researchers are working to develop them.
Be on the lookout for my next article, where I’ll introduce you to some of the faces of water splitting research here at the University of Michigan.
Read part two of “Water splitting” here.
About the author
 Jimmy is a 5th-year graduate student in the University of Michigan Department of Chemistry, exploring new chemical reactions to make photocatalysts for solar energy storage. He’s a southwestern Pennsylvania native and graduated from Duquesne Universtiy in Pittsburgh in 2011. Jimmy spends his off time at the roller hockey rink, playing involved board games, or annoying his cat. He also blogs chemistry and student issues at Tree Town Chemistry.
Jimmy is a 5th-year graduate student in the University of Michigan Department of Chemistry, exploring new chemical reactions to make photocatalysts for solar energy storage. He’s a southwestern Pennsylvania native and graduated from Duquesne Universtiy in Pittsburgh in 2011. Jimmy spends his off time at the roller hockey rink, playing involved board games, or annoying his cat. He also blogs chemistry and student issues at Tree Town Chemistry.
Read all posts by Jimmy here.

Solar is 1.7% of global power (https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy/renewable-energy/solar-energy.html) so a back-of-the-envelope calculation says you’re wrong by an order of magnitude or so. Also please don’t be an asshole in the comments.
LikeLike